Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

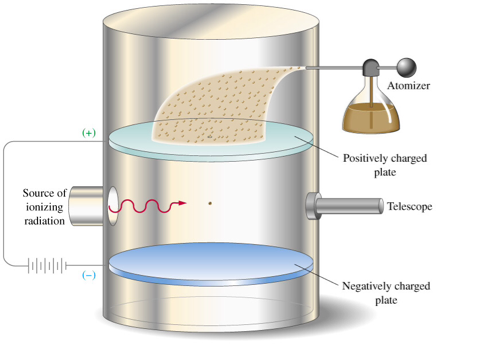
Millikan set up a pair of parallel conducting plates horizontally, one above the other, with a large electric field between them that could be adjusted. A fine mist of oil was sprayed into a chamber above the plates. Many of the droplets would become negatively charged as they picked up some small, unknown number of electrons as they passed through the nozzle. Some of the drops then fell through a hole in the top plate and drifted into the region between the two parallel plates. Lit from the side by an intense light, these drops glistened when the region was viewed through a microscope.
With the electric field turned off, Millikan could observe a falling drop and measure its terminal velocity. This measurement gave him the radius of the drop, and since he knew the density, he could determine the mass. He could then switch on the electric field, and adjust it so that the electric force just precisely balanced the force of gravity on the drop. Knowing the strength of the field and the mass of the drop, he could calculate the only unknown, the charge on the drop. This measurement was repeated many times, and often the same drop would be allowed to rise and fall in the apparatus again and again, as it picked up and shed electrons.