Science Report

Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

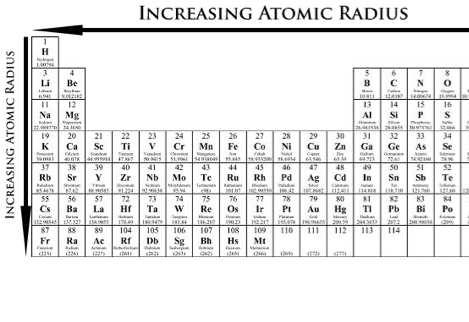
Atomic Radii

Atomic size gradually decreases from left to right across a period of elements. This is because, within a period or family of elements, all electrons are being added to the same shell. But, at the same time, protons are being added to the nucleus, making it more positively charged. The effect of increasing proton number is greater than that of the increasing electron number; therefore, there is a greater nuclear attraction. This means that the nucleus attracts the electrons more strongly, having the atom's shell pulled closer to the nucleus. The valence electrons are held closer towards the nucleus of the atom. As a result, the atomic radius decreases.
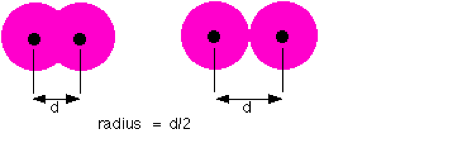
For atoms, the atomic radius is one-half the distance between the nuclei of two atoms is (just like a radius is half the diameter of a circle). However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Some are bound by covalent bonds in molecules, some are attracted to each other in ionic crystals, and others are held in metallic crystals. Nevertheless, it is possible for a vast majority of elements to form covalent molecules in which two like atoms are held together by a single covalent bond. The covalent radius of these molecules is often referred to as the atomic radius. This distance is measured in picometers. Going through each of the elements of the periodic table, patterns of the atomic radius can be seen.