Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


Chemical Test for Identication
Alkali Metals
Halogens
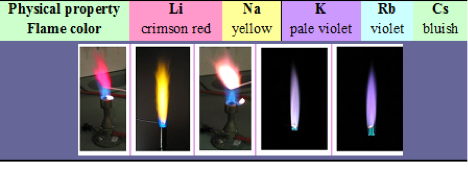
When the element is placed in a flame, each one gives off a different color based on the energy emitted giving wavelengths that are in the visible regions from the outer electron jumping to the highest energy level.
🔴 Chlorine – Turns damp litmus red, and then bleaches it.
🔴 Bromine – Bromine also turns damp litmus red and bleaches it but slower. When sodium hydroxide is added, bromine loses its colour.
🔴 Iodine – When starch solution is added, a blue/black colour forms.