Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

The difference between Polar and Nonpolar bonds
- Nonpolar covalent bonds are a type of bond that occurs when two atoms share a pair of electrons with each other. And Polar covalent bonding is a type of chemical bond where a pair of electrons is unequally shared between two atoms.
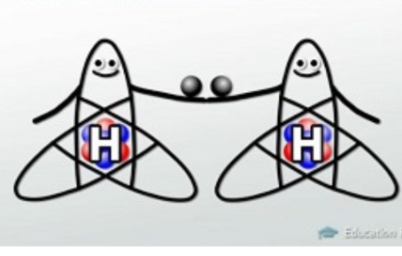
In a nonpolar covalent bond, the atoms share electrons equally with one another.
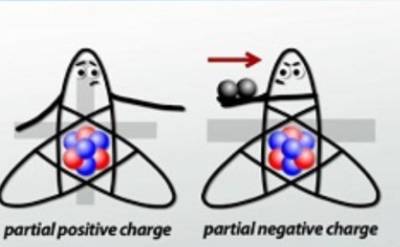
In a polar covalent bond, one atom spends more time with the electrons than the other