Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


Born September 6, 1766
John Dalton
Contribution-(Matter/Atomic Structure), proposed that all matter was composed of atoms, indivisible and indestructible building blocks. While all atoms of an element were identical, different elements had atoms of differing size and mass.
Eaglesfield Cumbria, United Kingdom
Experiments with gases that first became possible at the turn of the nineteenth century led John Dalton in 1803 to propose a modern theory of the atom based on the following assumptions.
1. Matter is made up of atoms that are indivisible and indestructible.
2. All atoms of an element are identical.
3. Atoms of different elements have different weights and different chemical properties.
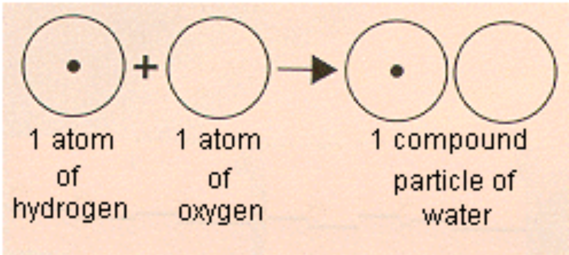
4. Atoms of different elements combine in simple whole numbers to form compounds.
5. Atoms cannot be created or destroyed. When a compound decomposes, the atoms are recovered unchanged.
Made his discovery in 1803