Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


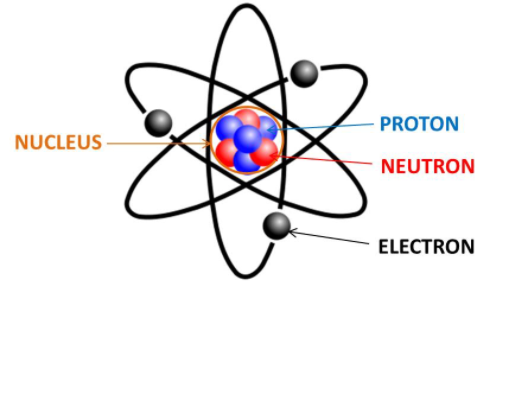
Atomic theory:
During James’ time: belief there were “nuclear electrons” (meaning electrons in the nucleus) along with additional protons.
Also in 1921, Ernest Rutherford proposed a new particle called the “neutron,” having a similar mass of a proton and being electrically neutral, but he had no evidence to support it.
In 1930, Walther Bothe and Herbert Becker did an experiment where they discovered photons.
Curious, James Chadwick tried these experiments out for himself. This is how James figured out that the neutral energy shooting back was not the photons but instead a neutron.
Atomic theory:
M A I A S A U R A
S T E G O S A U R U S

4

