Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

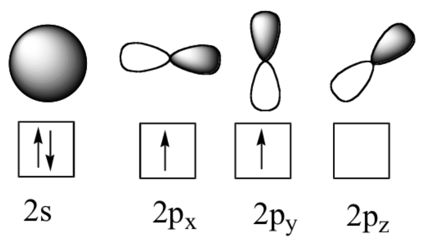
How To Represent Electronic Configuration
The arrows represents the electrons orbiting each sublevel. Arrows are drawn in opposite directions. As you can see each sublevel has a different shape. This is because the sublevel, s has a single orbital creating a spherical shape. However the p sublevel is made up of 3 identical dumbbell shaped orbitals, each of which sits on its own axis, 90* from the orbital previous.
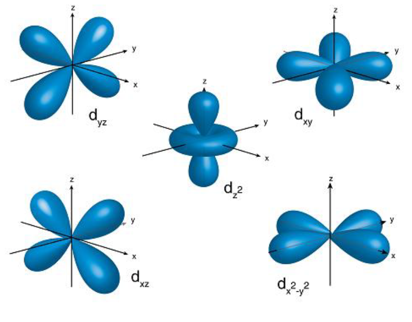
As you can see the sublevel d has 5 different shaped orbitals therefore can hold up to 10 electrons.