Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


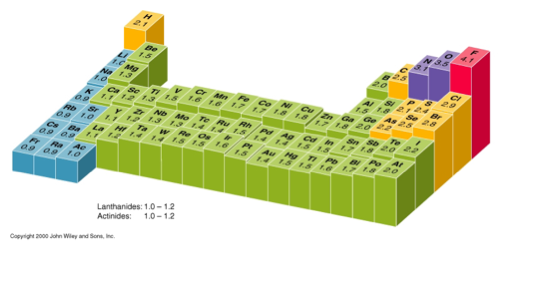
WHAT TREND IS DISPLAYED VERTICALLY?
The value decreases from top to bottom.
In an element group, the electronegativity decreases as atomic number increases, as a result of increased distance between the valence electron and nucleus (greater atomic radius).
An example of an electropositive (i.e., low electronegativity) element is cesium.
An example of a highly electronegative element is fluorine.
Moving left to right across the periodic table, electronegativity increases.
Moving top to bottom down the periodic table, elctronegativity decreases.
WHAT TREND IS DISPLAYED HORIZONTALLY?
The trend increases from left to right.
WHICH DIRECTION OF MOVEMENT HAS A BIGGER RELATIVE CHANGE?
The horizontal trend has a bigger change.