Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

Bohr explained how electrons could jump from one electron to another by emitting or absorbing energy in fixed quantities. For example, if an electron jumps one bit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. Conversely, when the electron jumps to a larger orbit, it must absorb a quantum of light equal in energy to the difference in orbit
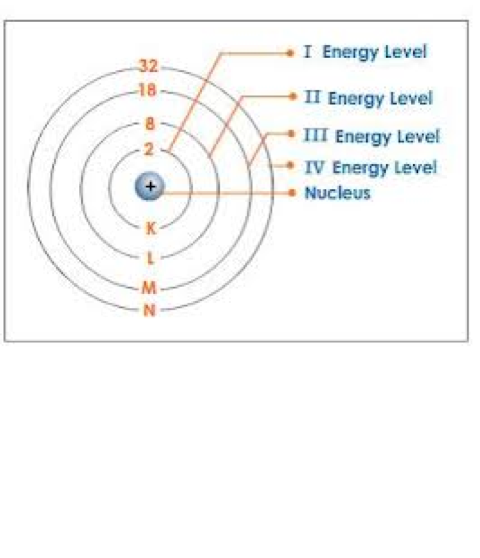
The evidence that led to a change in the previous model: