Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

Electron affinity

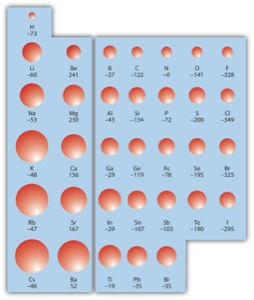
Electron affinity
1:the electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion.
2:electron affinity increase across a periodic table.
3:why, because the more nuclear charge an atom has the more likely it is to attract an electron.therefore, as nuclear charge increase from left to right within a period so too does electron affinity.