Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

What was the evidence (data) in his experiment?
Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of "bodies much smaller than atoms" that he calculated as having a very large value for the charge-to-mass ratio.
Thomson also found that the mass-to-charge ratio for cathode rays turned out to be far smaller than that of a charged hydrogen atom--more than one thousand times smaller. Either the cathode rays carried an enormous charge (as compared with a charged atom), or else they were amazingly light relative to their charge.
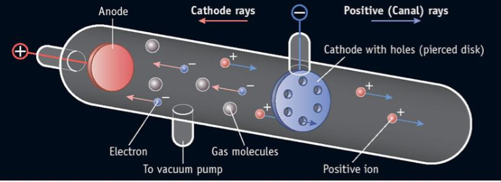
The experiments also provided evidence that a atom contains negatively charged particles.