Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


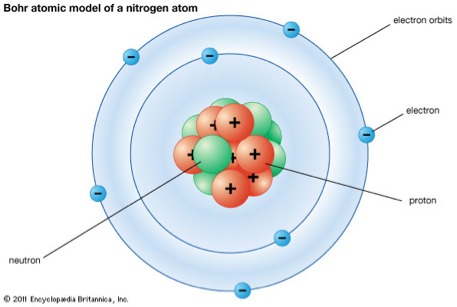
The Experiment of Bohr
Bohr was building off the work of his mentor Rutherford, but found something wrong. He then began to experiment to find the truth.Bohr was able to find this by using math with hydrogen gas. He was using Planck's constant (6.6x10^-34 joules). With this Bohr found out the energy value of the hydrogen atom. He though that the way the electrons move was that at a set amount of energy. They were quantized. Then he assumed that electrons fallow the law of classic mechanics and that means that they orbit around the nucleus. Like how or solar system dose around the sun.