Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

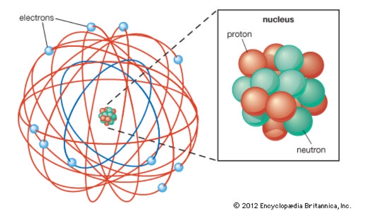
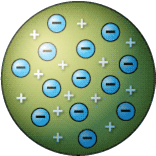
• Rutherford disproved the plum pudding model with his gold foil experiment.
• Since most of the particles passed straight through the foil, he confirmed that atoms are mostly made of empty space.
• Because only a small number of particles were deflected at large angles, that meant there was a central positive charge- the nucleus.
• He claimed that electrons revolved in a circular motion around the nucleus, much like a solar system.
CONCLUSION