Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

J.J. THOMSON'S MODEL
Before Rutherford's discovery, people believed that J.J. Thomson's Plum Pudding Model was an accurate representation of all atoms.
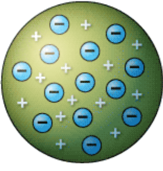
By conducting a gold foil experiment, Rutherford knew Thomson was wrong because he believed there was a central positive charge in the atom.
Plum Pudding Model:
• Described an atom as being a positively charged body that contained free-floating, negatively charged electrons.
• Stated that the overall charge of an atom was neutral since the negative and positive charges canceled each other out.