Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow



Ionic Radius on the periodic table


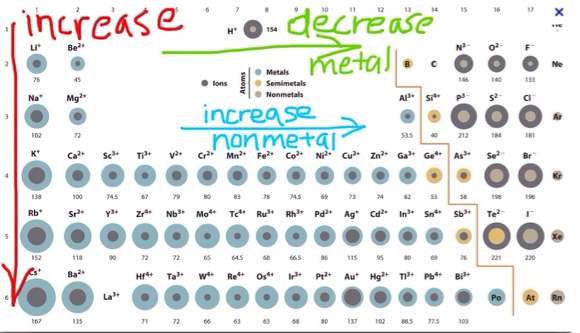

Why increase as you go down?
As you go down a group, you gain shells, just like in atomic radii.
Why decrease?
When metals lose electrons, they also lose their shells so the ion gets smaller. When the non metals gain electrons, it cancels out the proton's pull so the ion gets bigger.
