Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


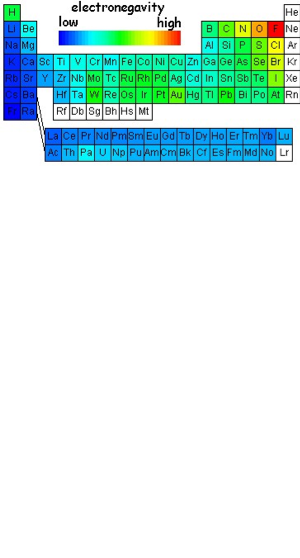
In my words: atoms tend to attract electrons, they increase as the move left to right, except noble gases they decrease as they move down the column.
Electronegavity
Definition
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7.