Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


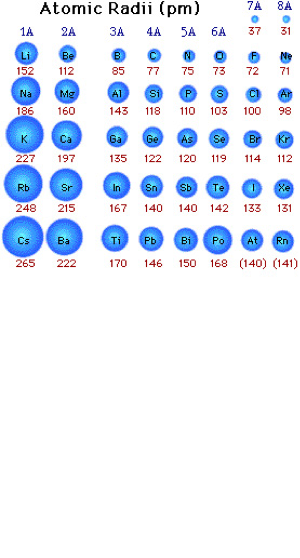
Ionic radii
In my words: for anions that gain electrons as the ionic radii increases compared to the atomic radii, for cations they lose electrons as the ionic radii decreases.
Definition: The ionic radius is the measure of an atom's ion in a crystal lattice. Values for ionic radius are difficult to obtain and tend to depend on the method used to measure the size of the ion. A typical value for an ionic radius would be from 30 pm (0.3 Å) to 200 pm (2 Å). Ionic radius may be measured using x-ray crystallography or similar techniques.