Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

Why the boiling point changes as you go down the fractional column?
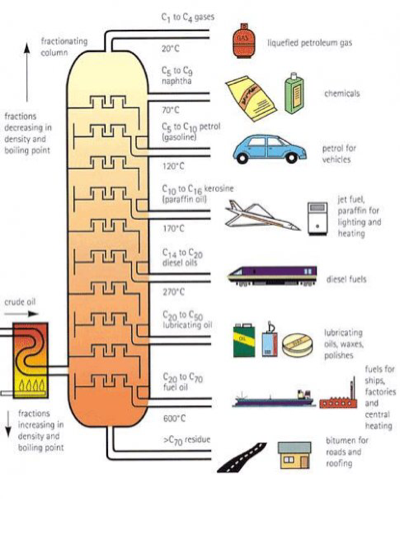
Oil in the fractional column is exposed to different temperatures of heat starting from the hottest at the bottom. The substance that has the highest boiling point will start at the bottom as it needs the highest temperature to evaporate and the highest substance has the lowest boiling point. Using this method, we can extract the different chemicals from crude oil.