Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

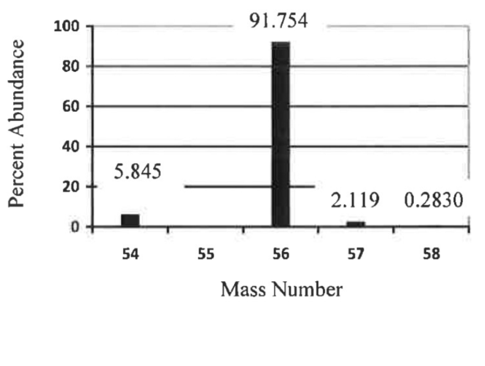
A pure sample of an element was vaporized and injected into a mass spectrometer and the data was plotted on the graph below. The mass values for the isotopes were found to be: A-56 (55.935 amu), A-54 (53.940 amu), A-57 (56.935 amu), and A-58 (57.933 amu). Find the average atomic mass and identify the element.
Lecture 1, Question 2

Chris