Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


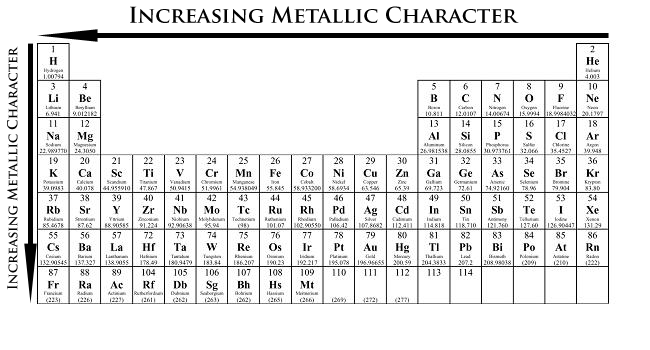
-Increase from right to left because the attraction between valence electrons and the nucleus is weaker
-Increase as you move down because the atomic size is bigger
-A bigger atomic size has to do with the outer shells being farther away
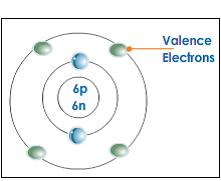
-When the electrons on the valence shell have a little attraction to the nucleus it can loose electrons easily
-Nonmetallic characters deal with the ability to gain electrons
Metallic Character
-How easily an atom can loose electron