Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


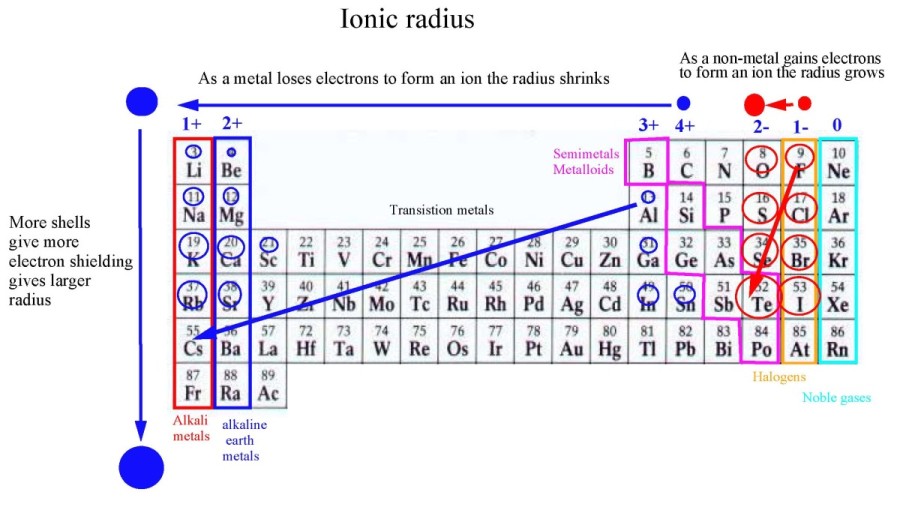
Ionic Radii
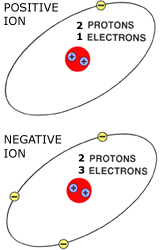
-Measure of the radius of an ion
-Measured in anions and cations
-Anions: are negative which means they gain electrons
-Cations: are positive which means they loose electrons
-Increase going down to the left of the periodic table
-When it looses electrons the radius gets smaller and vice versa
-Positive ions are smaller than their uncharged atoms
-Negative ions are larger than their atoms