Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


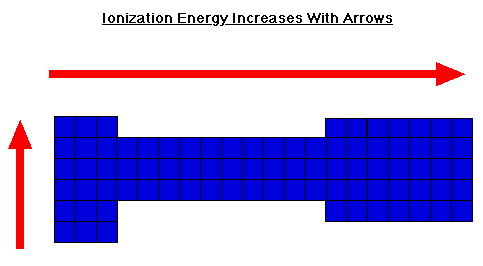
-They increase as they go up and to the right
-Usually showed in Kg/mol
-Easier to take electrons from unstable elements; Ex- Ca
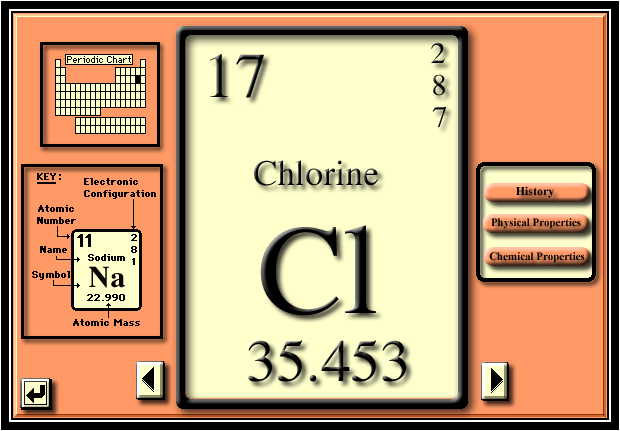
-Stable elements like Cl is harder to take electrons from
-Elements close to each other on the periodic table form covalent bonds; Ex:oxygen & carbon
Ionization Energy
-Amount of an energy required to remove an electron

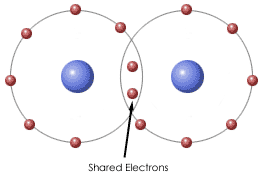
Covalent Bond