Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

Akaline Earth Metal Slide
Alkaline Earth Metals include the following metals:
-Beryllium ( Be )
-Magnesium ( Mg )
-Calcium ( Ca )
-Strontium ( Sr )
-Barium ( Ba )
-Radium ( Ra )
Alkaline Earth Metals are in fact:
-Luster
-Conductive
-Malleable
-Ductile
-Reactive
Alkaline Earth Metals have 2 outer electrons
Magnesium is the lightest metal that can be used to build things although its use as a structual materials limited since it burns at a relatively low temperature
Calcium is seldom found as a free metal and it is in bones as calcium phosphate
Radium is radioactive
A significant medical use of Barium is to enhance x-ry images of the intestinal tract.
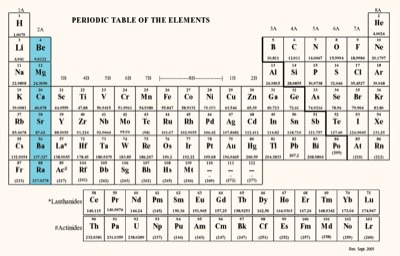
Alkaline Earth Metals are located in group 2
Alkaline Earth Metals lose electrons while bonding and become positive

Beryllium rock -->