Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

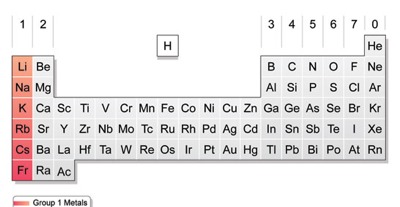
Alkali Metals include the following metals:
Alkali Metal Slide
-Lithium ( Li )
-Sodium ( Na )
-Potassium ( K )
-Rubidium ( Rb )
-Caesium ( Cs )
-Francium ( Fr )
Alkali Metals are in fact:
-Malleable
-Ductile
-Conductive
-And they have luster
But how reactive are they?
Alkali Metals are extremely reactive and do not occur freely in nature.
Alkali Metals do in fact react to air or water if exposed to it, so to keep it under control they store these metals in oil.
These metals only have one outer electron.
Alkali Metals are located in group number 1 in the periodic table.
Alkali Metals had to lose an electron while bonding to become positive.
From my knowledge, all living things need Potassium ( K ) to survive.
Francium is a very rare element that wasn't even discovered until 1939.