Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

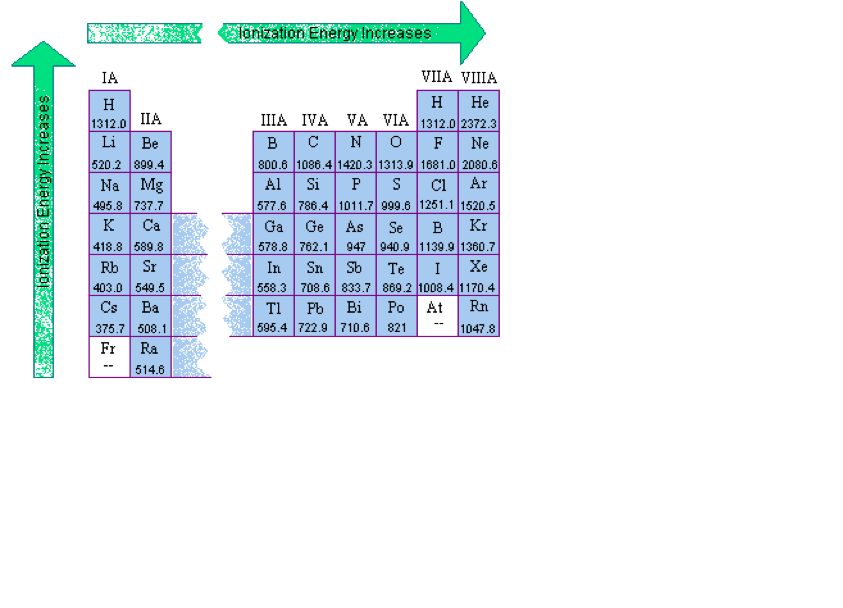
Ionization Energy
As one progresses down a group on the periodic table, the ionization energy will likely decrease since the valence electrons are farther away from the nucleus and experience a weaker attraction to the nucleus's positive charge. There will be an increase of ionization energy from left to right of a given period and a decrease from top to bottom.