Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

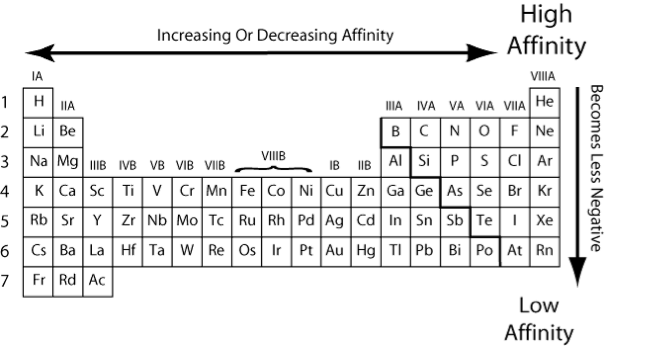
Electron Affinity
The electron affinity of an atom can be described either as the energy gained by an atom when an electron is added to it, or conversely as the energy required to detach an electron from a singly charged anion. The sign of the electron affinity can be quite confusing, as atoms that become more stable with the addition of an electron (and so are considered to have a higher electron affinity) show a decrease in potential energy; i.e. the energy gained by the atom appears to be negative. For atoms that become less stable upon gaining an electron, potential energy increases, which implies that the atom gains energy.