Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

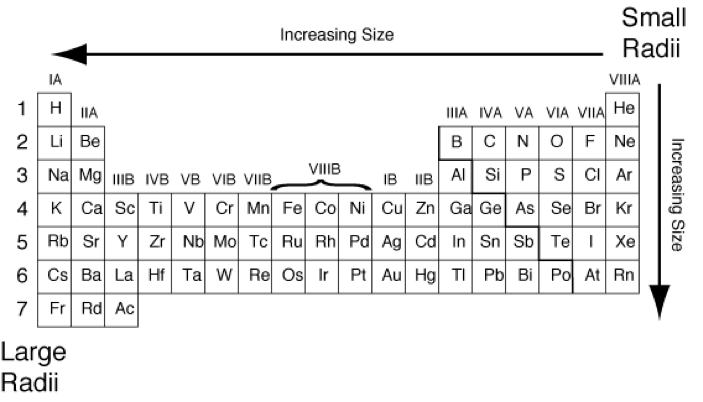
Atomic radius
The atomic radii tends to decrease across a period from left to right. The atomic radius usually increases while going down a group due to the addition of a new energy level (shell). However, diagonally, the number of electrons has a larger effect than the sizeable radius.