Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

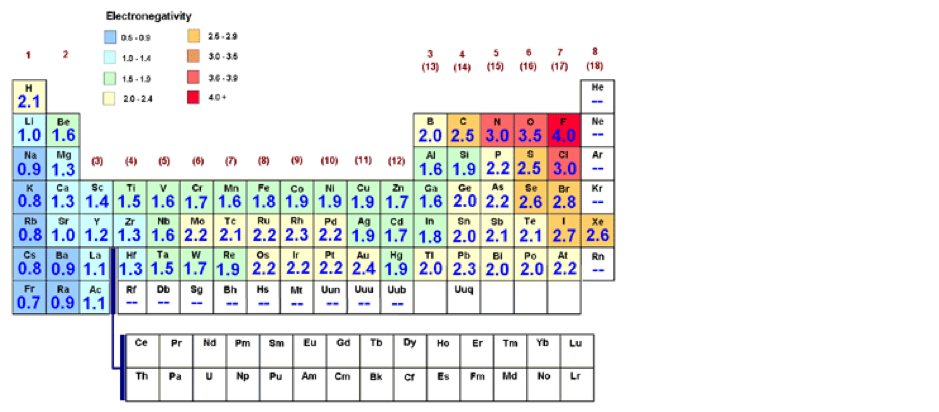
Electronegativity
Electronegativity is a measure of the ability of an atom or molecule to attract pairs of electrons in the context of a chemical bond. The type of bond formed is largely determined by the difference in electronegativity between the atoms involved, using the Pauling scale. Trend-wise, as one moves from left to right across a period in the periodic table, the electronegativity increases due to the stronger attraction that the atoms obtain as the nuclear charge increases. Moving down in a group, the electronegativity decreases due to the longer distance between the nucleus and the valence electron shell.