Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

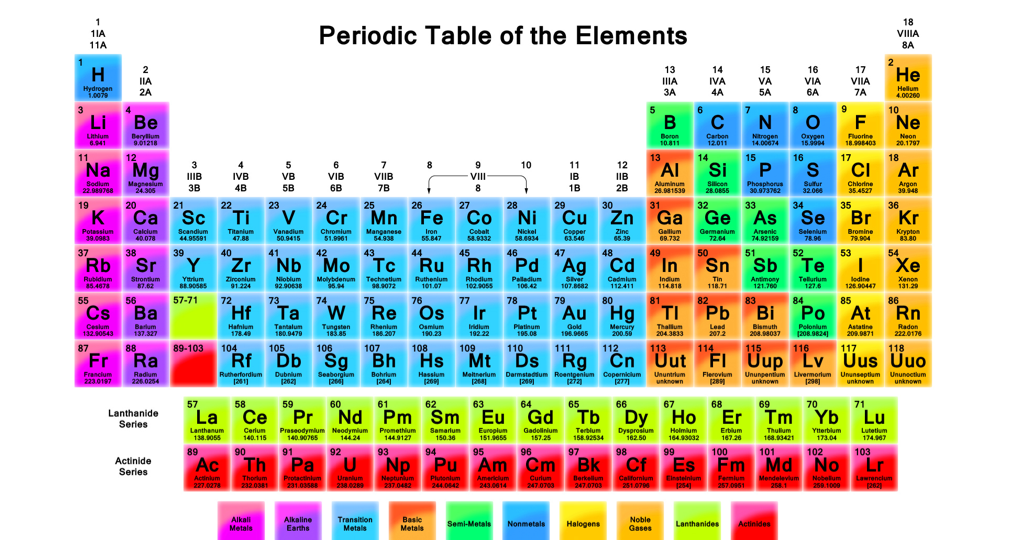
Periodic Trends
All periodic trends of the chemicals are based on Coulomb's law . As distance from the protons in the nucleus to the valence electrons increases values associated with attributes such as electron affinity, ionization energy, and electronegativity decrease.