Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

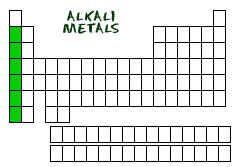
Alkali Metals
The Alkali metals are Lithium, Sodium, Potassium, Rubidium, Cesium, and Francium
Alkali metals are malleable.
Alkali metals are ductile.
Alkali metals do have luster.
Alkali metals are good conductors.
Alkali metals are very reactive to water ad they will explode if they have contact to water.
No, Alkali metals do not occur in nature naturally.
Because Alkali metals are reactive to water and air they are stored in oil to prevent it getting to the metals.
Alkali metals have one valence electron.
Alkali metals have one valence electron and that means they are one electron from being complete so they wan to get another electron to become positive.
Sodium and Potassium need living things to live because they are nutrients.
Francium is very rare which means it is the rarest Alkali metal it was not discovered until 1939.

Location of Alkali Metals on Periodic Table
Lithium, one of the Alkali metals
The group number of the Alkali metals is Group 1.