Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

Nonmetals
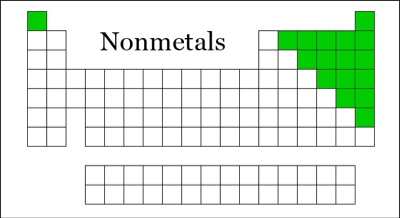
The Nonmetals are Hydrogen, Helium, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Phosphorus, Sulfur, Chlorine, Argon, Selenium, Bromine, Krypton, Iodine, Xenon, Astatine, Radon, Ununseptium, Ununoctium.
Nonmetals are not good conductors.
They have no luster.
They are not malleable or ductile.
Nonmetals become reactive as they gain more and more electrons.
Nonmetals have 2 valence electrons.
Nonmetals are either gases or brittle because they have low densities.
Nonmetals gain electrons when they bond.

Hydrogen is very reactive it bonds with other electron to create hydrides, which are the different additions of elements.
They are found in groups 14 - 16 and Hydrogen is in group 1.
They tend to become negative when gaining electrons.
Location of Nonmetals on Periodic Table
You bodies mass is mainly oxygen carbon hydrogen, nitrogen and phosphorus.