Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

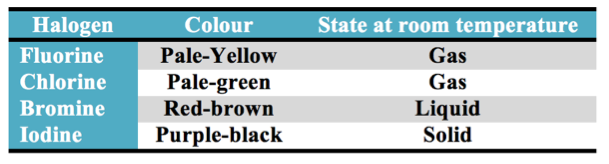
Halogens
The Halogen Elements are Fluorine, Chlorine, Bromine, Iodine, and Astatine.
Halogens are not good conductors of heat or electricity.
Halogens do not have luster.
They are not malleable or ductile.
Halogens are reactive they need one more valence electron to fill the outer shell.
Halogens have 7 valence electrons.
Halogens have chemical properties that allow them to create salts.
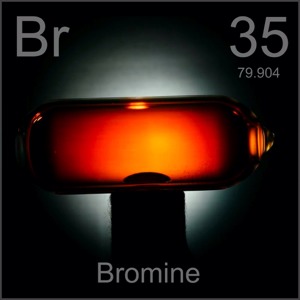
Bromine is the only nonmetal that is solid at room temperature.
At room temperature Iodine is a solid.
The Halogens group number is 17.