Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow

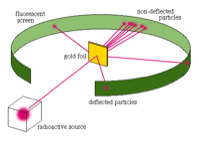
Alpha rays were also discovered around this time. Rutherford thought it would be a good idea to throw these alpha rays at the atom to penetrate the surface and see what was in the inside.
He used Radium as his source of alpha particles, and shined it at the atoms gold foil.
The actual results deviated from Rutherford's prediction.
Ernest Rutherford