Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


Then Einstein stepped in!
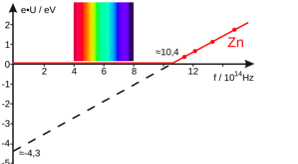
Max Planck showed that if you vibrate atoms strong enough, you can measure the energy only in discrete units. He called these energy packets 'quanta.' However, Einstein thought that the quanta behaved like discrete particles, and named these light particles 'photons.'
That was in 1900, but 5 years later, Einstein made a ground-breaking discovery called the "photoelectric effect." This explained that light absorption can release electrons from atoms.
