Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


Niels Bohr took charge!
After Rutherford determined that the nucleus had a positive charge that attracted the negatively charged electrons, physicists argued against this theory saying that there were flaws in his argument.
In 1912, the Danish physicist Neils Bohr challenged all previous models of the atom with one that actually explained the rules behind his ideas.
He said, "Here's some rules that seem impossible, but they describe the way atoms operate."
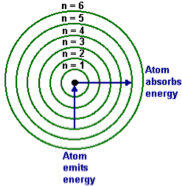
RULE 1:
Electrons can only orbit at certain allowed distances from the nucleus.
RULES 2:
Atoms radiate energy when an electron jumps from a higher-energy orbit to a lower-energy orbit. Also, an atom absorbs energy when an electron gets boosted from a low-energy orbit to a high-energy orbit.