Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


Moving on
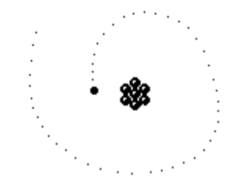
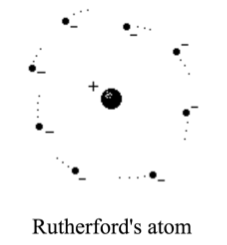
Rutherford discovered that most of the alpha particles smoothly went through the foil. It was only the occasional alpha particle that took a sharp deviation from its path. He concluded that the atoms must get scattered by tiny parts of positively charged matter, and so he thought that the electrons must exist somewhere within this empty space.
And that's when he came up with this theory: