Sign up for FlowVella
Sign up with FacebookAlready have an account? Sign in now
By registering you are agreeing to our
Terms of Service
Loading Flow


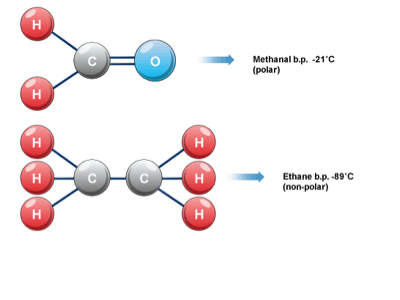
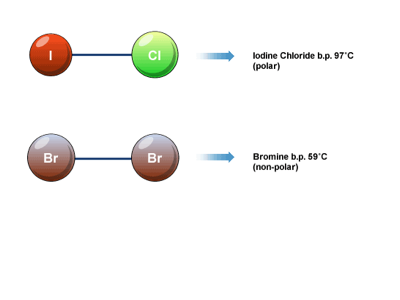
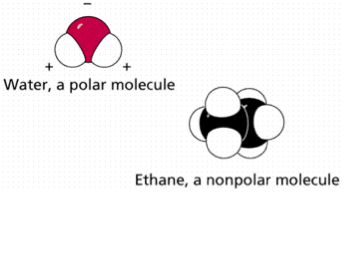
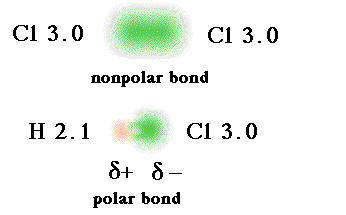
15. Nonpolar molecules are electrically balanced because they do not have separate positive and negative poles of charge. Polar molecules result when there is unequal electron pair sharing. Carbon dioxide and water can demonstrate how molecular shape and their relative electron-attracting abilities of atoms determine whether a covalently bonded molecule is polar or nonpolar. In carbon dioxide, carbon shares four electron pairs with two oxygen atoms. Oxygen is very electronegative and attracts the shared electrons more strongly than carbon. However, because the carbon dioxide molecule is linear and symmetrical, the electron-pulling ability of one oxygen atom is offset by the other. The shared electrons orbit the entire molecule and carbon dioxide is a nonpolar molecule.